Let’s talk about cosmetic packaging. They say the devil’s in the details, and in the world of cosmetics, the details are all that stand between a masterpiece and a lawsuit. You’ve seen those tiny letters on the back of a bottle, the fine print that nobody reads but everyone relies on. Let me tell you—those words aren’t just there to fill space. They’re the law.
Cracking the Code of Cosmetic Packaging
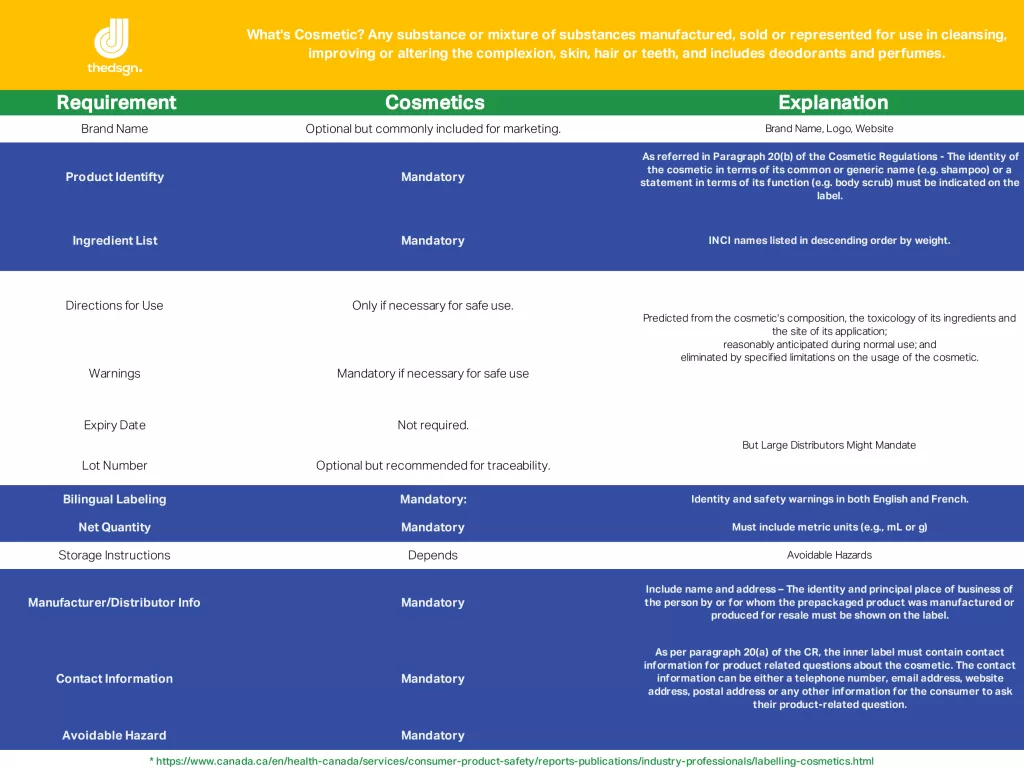
Cosmetic label is a symphony of regulations and expectations, and if you’re a manufacturer or an indie brand dreaming big, it’s a tune you’d better learn to play. So, what exactly do you need on that label? Buckle up; we’re diving deep.
Ingredients: The INCI List Is Your Best Friend
No shortcuts here, folks. Every single ingredient must be disclosed using its International Nomenclature Cosmetic Ingredient (INCI) name. Yes, even that trendy herb extract you thought could slide under the radar. Transparency isn’t just ethical—it’s the law. Consumers have a right to know what they’re slathering on their skin, and in North America, there’s no room for mystery.

Batch Numbers: To Code or Not to Code?
Here’s where things get a bit looser. North America doesn’t require batch numbers on cosmetic labels, but let’s be real. If you’re not tracking your batches, you’re playing with fire. One slip-up, one recall, and you’ll wish you had a way to trace every product back to its origin. Don’t skip this—it’s your safety net.
Use-By Dates: The Myth of Forever Fresh
Unlike in the EU, North America doesn’t demand an expiration date on cosmetic products. But should you include one? Absolutely. Nothing lasts forever—not even that high-performance face cream. A shelf-life indicator shows customers you’re committed to their safety and satisfaction. It’s not legally binding, but it’s a trust-building move that pays dividends.
Warnings: Don’t Play With Fire
Warnings are the unsung heroes of cosmetic labels. They’re there to protect your customers from misuse and your brand from lawsuits. Got a product with active ingredients like retinol? Make it clear that sun protection is a must. Is your glitter-loaded eyeshadow not safe for use around the eyes? Spell it out. These disclaimers aren’t just CYA tactics—they’re lifesavers.
Canada: A World of Its Own
Oh, Canada. We march to the beat of a slightly different drum. If you’re selling cosmetics here, be prepared for bilingual labeling. English and French must appear side by side, making your packaging design a bit of a puzzle – and that’s why we are here to help. Plus, Health Canada has its own ingredient hotlist—a compilation of substances that are restricted or outright banned. Check it twice, or you might find your product seized at the border.
OTC vs. Cosmetics: Worlds Apart
If you’re dabbling in over-the-counter (OTC) products—think acne treatments or fluoride toothpaste—you’ve entered a whole new regulatory realm. These products are classified as drugs, meaning you’ll need a Drug Identification Number (DIN) in Canada or an equivalent registration in the U.S. Your labeling game needs to level up with precise dosage instructions, active ingredient percentages, and more. It’s not cosmetic; it’s clinical.
The Power of a Well-Labeled Product
A good cosmetic label is like a love letter to your customer. It tells them what’s inside, how to use it, and why they can trust you. Sure, the rules are a labyrinth, but they’re also a roadmap to success. If you’re in this game for the long haul, it’s worth every ounce of effort.
The Future of Labeling: It’s Not Just Paper Anymore
Smart packaging isn’t science fiction anymore—it’s breathing down our necks. QR codes linking to full ingredient breakdowns, augmented reality tutorials, blockchain batch tracking. The label of tomorrow isn’t just a sticker; it’s a digital doorway to everything your customer needs to know. But here’s the truth bomb: all the fancy tech in the world won’t save you if your basic compliance isn’t airtight.
Remember those FDA cosmetic labeling rules I mentioned? They’re evolving too. The regulators aren’t blind to innovation, but they’re moving at their own pace. Your job is to stay ahead of the curve while keeping one foot firmly planted in current compliance.
Manufacturing Realities: When Theory Meets Production Line
Let’s talk about what happens when beautiful design meets manufacturing constraints. Your gradient-color label might look fantastic on screen, but can your printer handle it? Will those metallic inks pass stability testing? Every decision impacts your bottom line, and in this business, margins are thinner than that serum you’re selling.
Small batch producers, this is where you need to pay attention. Your labels need to work harder because you can’t afford mistakes. Every misprint is money down the drain, and in small-batch production, those dollars hit harder.
The International Dance: Cross-Border Compliance
Thinking of selling internationally? Buckle up. Each market adds layers of complexity to your labeling requirements. The EU wants your PAO (Period After Opening) symbol. Asia has its own character requirements. And Canada? They’re particular about their French translations—and no, Google Translate won’t cut it.
Want More?
Download our detailed checklist and watch the video guide to unravel every nuance of cosmetic labeling. Whether you’re starting out or scaling up, this is your ultimate toolkit for compliance and clarity.
Labels are more than ink on paper. They’re promises—to your customers, to the law, and to your brand’s future. Make them count.